Climate Change Impacts on the Marine Cycling of Biogenic Sulfur: A Review
Abstract
:1. Introduction
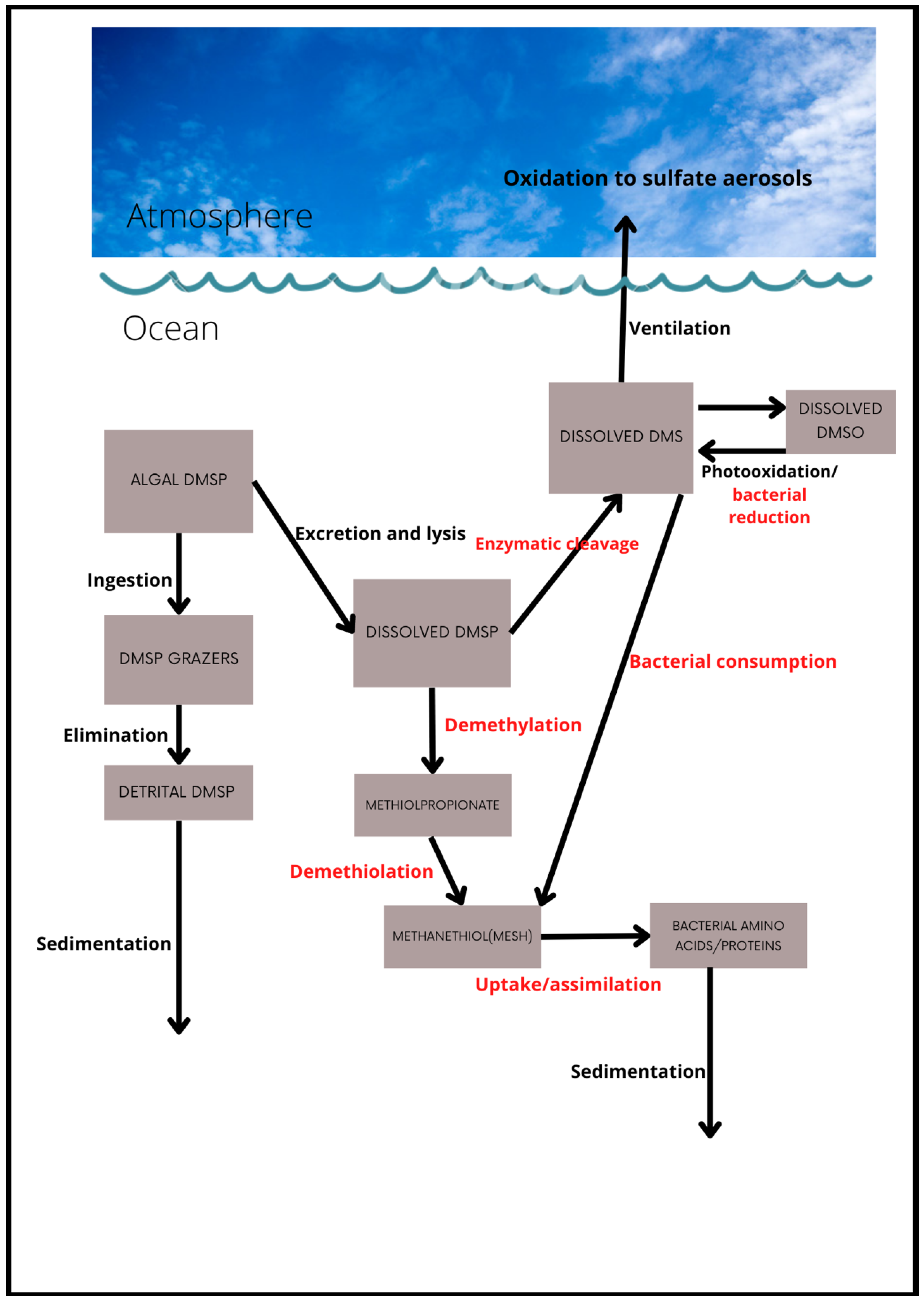
2. The Marine DMS Cycle
2.1. Key Food Web Processes
2.2. Role of Heterotrophic Organisms
2.3. Impacts of Changes to Ocean Temperature and pCO2
2.4. Modeling
2.5. Laboratory Experiments
3. Projected Ocean Warming and Acidification
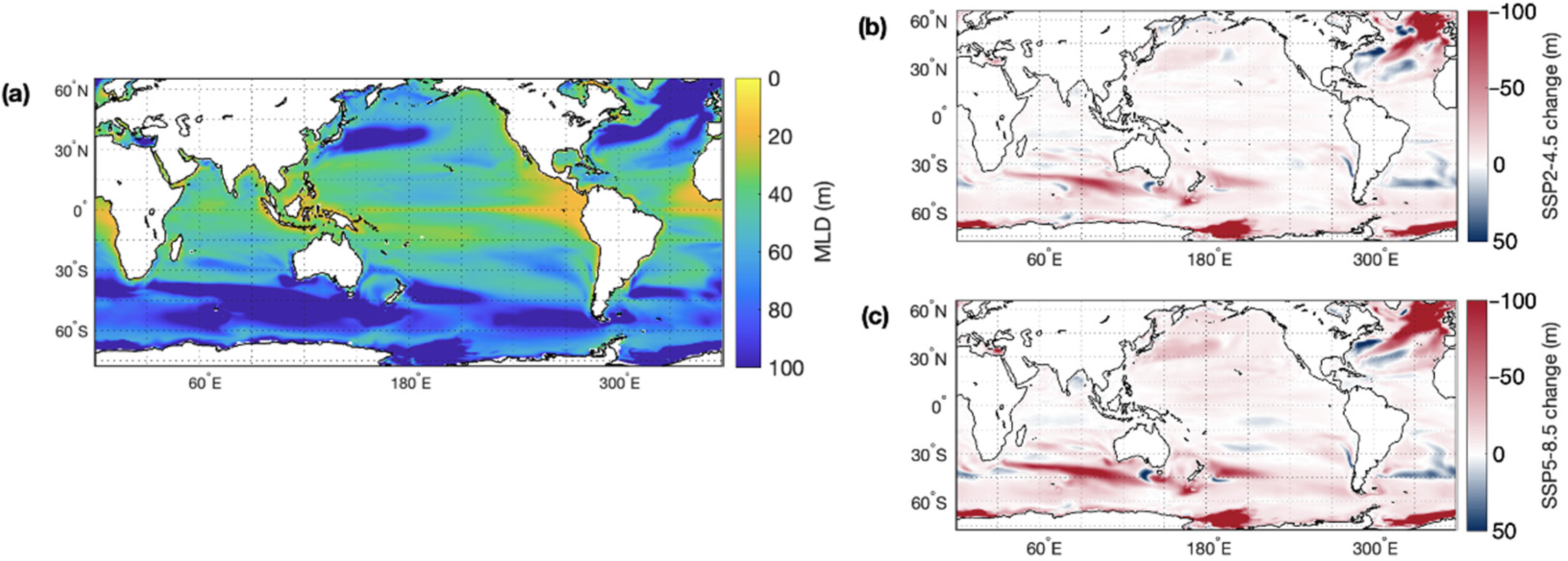
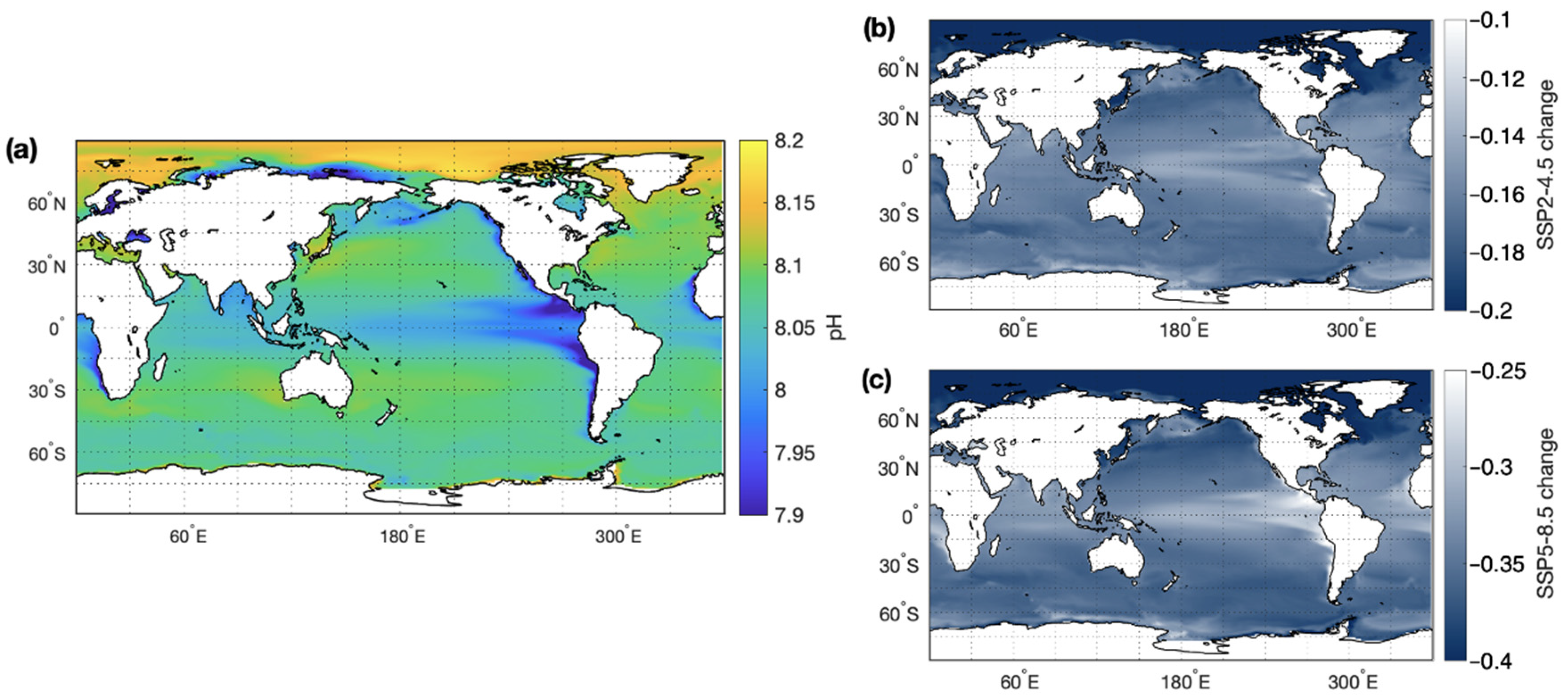
3.1. Global CMIP6 Climate Projections
3.1.1. Ocean Warming
3.1.2. Ocean Acidification
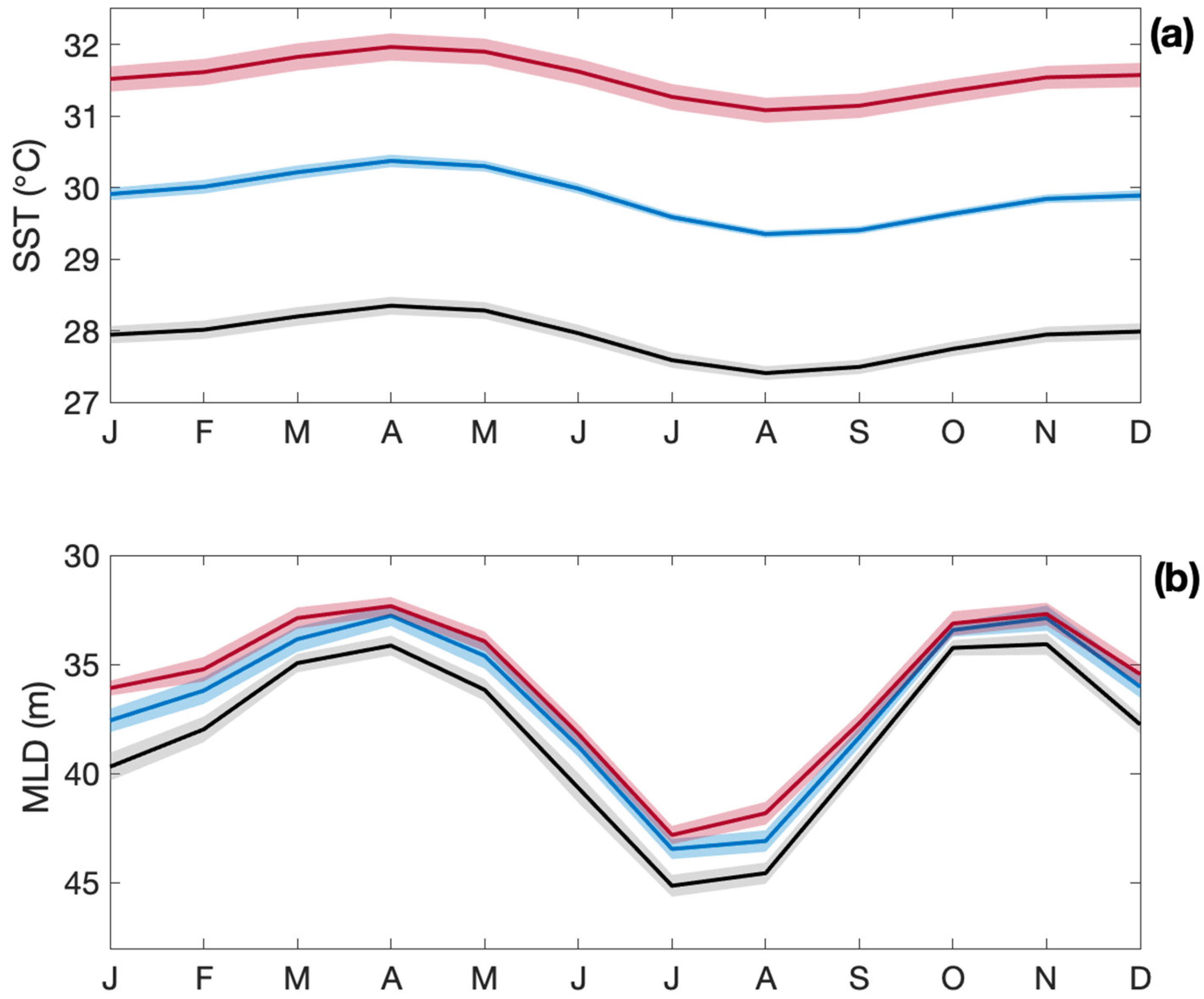
3.2. Impacts on DMS Cycling in the Polar Oceans
3.3. Impacts on the Tropical Ocean and Coral Reefs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreae, M.O.; Crutzen, P.J. Atmospheric aerosols: Biogeochemical sources and role in atmospheric chemistry. Science 1997, 276, 1052–1058. [Google Scholar] [CrossRef]
- Shaw, G.E. Bio-controlled thermostasis involving the sulphur cycle. Clim. Chang. 1983, 5, 297–303. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Brévière, E.H.G.; Bakker, D.C.E.; Bange, H.W.; Bates, T.S.; Bell, T.G.; Boyd, P.W.; Duce, R.A.; Garçon, V.; Johnson, M.T.; Law, C.S.; et al. Surface ocean-lower atmosphere study: Scientific synthesis and contribution to Earth system science. Anthropocene 2015, 12, 54–68. [Google Scholar] [CrossRef]
- Ayers, G.P.; Cainey, J.M. The CLAW hypothesis: A review of the major developments. Environ. Chem. 2007, 4, 366–374. [Google Scholar] [CrossRef]
- Liss, P.S.; Marandino, C.A.; Dahl, E.E.; Helmig, D.; Hintsa, E.J.; Hughes, C.; Johnson, M.T.; Moore, R.M.; Plane, J.; Quack, B. Short-lived trace gases in the surface ocean and the atmosphere. In Ocean-Atmosphere Interactions of Gases and Particles; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–54. [Google Scholar]
- Wang, W.L.; Song, G.; Primeau, F.; Saltzman, E.S.; Bell, T.G.; Moore, J.K. Global ocean dimethyl sulfide climatology estimated from observations and an artificial neural network. Biogeosciences 2020, 17, 5335–5354. [Google Scholar] [CrossRef]
- Chin, M.A.; Jacob, D.J. Anthropogenic and natural contributions to tropospheric sulfate: A global model analysis. J. Geophys. Res.-Atmos. 1996, 101, 18691–18699. [Google Scholar] [CrossRef]
- Kilgour, D.B.; Novak, G.A.; Sauer, J.S.; Moore, A.N.; Dinasquet, J.; Amiri, S.; Franklin, E.B.; Mayer, K.; Winter, M.; Morris, C.K. Marine gas-phase sulfur emissions during an induced phytoplankton bloom. Atmos. Chem. Phys. 2022, 22, 1601–1613. [Google Scholar] [CrossRef]
- Smith, S.J.; van Aardenne, J.; Klimont, Z.; Andres, R.J.; Volke, A.; Delgado Arias, S. Anthropogenic sulfur dioxide emissions: 1850–2005. Atmos. Chem. Phys. 2011, 11, 1101–1116. [Google Scholar] [CrossRef]
- Mitchell, J.F.; Johns, T.; Gregory, J.M.; Tett, S. Climate response to increasing levels of greenhouse gases and sulphate aerosols. Nature 1995, 376, 501–504. [Google Scholar] [CrossRef]
- Westervelt, D.M.; Horowitz, L.W.; Naik, V.; Golaz, J.C.; Mauzerall, D.L. Radiative forcing and climate response to projected 21st century aerosol decreases. Atmos. Chem. Phys. 2015, 15, 12681–12703. [Google Scholar] [CrossRef]
- Aas, W.; Mortier, A.; Bowersox, V.; Cherian, R.; Faluvegi, G.; Fagerli, H.; Hand, J.; Klimont, Z.; Galy-Lacaux, C.; Lehmann, C.M.B.; et al. Global and regional trends of atmospheric sulfur. Sci. Rep. 2019, 9, 953. [Google Scholar] [CrossRef]
- Quinn, P.K.; Bates, T.S. The case against climate regulation via oceanic phytoplankton sulphur emissions. Nature 2011, 480, 51–56. [Google Scholar] [CrossRef]
- Novak, G.A.; Fite, C.H.; Holmes, C.D.; Veres, P.R.; Neuman, J.A.; Faloona, I.; Thornton, J.A.; Wolfe, G.M.; Vermeuel, M.P.; Jernigan, C.M.; et al. Rapid cloud removal of dimethyl sulfide oxidation products limits SO2 and cloud condensation nuclei production in the marine atmosphere. Proc. Natl. Acad. Sci. USA 2021, 118, e2110472118. [Google Scholar] [CrossRef]
- Fung, K.M.; Heald, C.L.; Kroll, J.H.; Wang, S.; Jo, D.S.; Gettelman, A.; Lu, Z.; Liu, X.; Zaveri, R.A.; Apel, E.C.; et al. Exploring dimethyl sulfide (DMS) oxidation and implications for global aerosol radiative forcing. Atmos. Chem. Phys. 2022, 22, 1549–1573. [Google Scholar] [CrossRef]
- Keller, M.D.; Bellows, W.K.; Guillard, R.L. Dimethyl Sulfide production in marine phytoplankton. In Biogenic Sulfur in the Environment; Saltzman, E.S., Cooper, W.J., Eds.; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef]
- Guibert, I.; Bourdreux, F.; Bonnard, I.; Pochon, X.; Dubousquet, V.; Raharivelomanana, P.; Berteaux-Lecellier, V.; Lecellier, G. Dimethylsulfoniopropionate concentration in coral reef invertebrates varies according to species assemblages. Sci. Rep. 2020, 10, 9922. [Google Scholar] [CrossRef]
- Reisch, C.R.; Moran, M.A.; Whitman, W.B. Bacterial catabolism of dimethylsulfoniopropionate (DMSP). Front. Microbiol. 2011, 2, 172. [Google Scholar] [CrossRef]
- Gregory, G.J.; Boas, K.E.; Boyd, E.F. The organosulfur compound dimethylsulfoniopropionate (DMSP) is utilized as an osmoprotectant by Vibrio species. Appl. Environ. Microbiol. 2020, 87, e02235-20. [Google Scholar] [CrossRef]
- Fernandez, E.; Ostrowski, M.; Siboni, N.; Seymour, J.R.; Petrou, K. Uptake of Dimethylsulfoniopropionate (DMSP) by Natural Microbial Communities of the Great Barrier Reef (GBR), Australia. Microorganisms 2021, 9, 1891. [Google Scholar] [CrossRef]
- Ainsworth, T.; Krause, L.; Bridge, T.; Torda, G.; Raina, J.-B.; Zakrzewski, M.; Gates, R.D.; Padilla-Gamiño, J.L.; Spalding, H.L.; Smith, C. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. ISME J. 2015, 9, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; Gabric, A.J.; Cropp, R.; Woodhouse, M.T. Dimethylsulfide (DMS), marine biogenic aerosols and the ecophysiology of coral reefs. Biogeosciences 2020, 17, 2181–2204. [Google Scholar] [CrossRef]
- Raina, J.B.; Tapiolas, D.M.; Foret, S.; Lutz, A.; Abrego, D.; Ceh, J.; Seneca, F.O.; Clode, P.L.; Bourne, D.G.; Willis, B.L.; et al. DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature 2013, 502, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Haydon, T.D.; Seymour, J.R.; Suggett, D.J. Soft corals are significant DMSP producers in tropical and temperate reefs. Mar. Biol. 2018, 165, 109. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Puglisi, M.P. DMSP in marine macroalgae and macroinvertebrates: Distribution, function, and ecological impacts. Aquat. Sci. 2007, 69, 394–402. [Google Scholar] [CrossRef]
- Sanchez, K.J.; Chen, C.-L.; Russell, L.M.; Betha, R.; Liu, J.; Price, D.J.; Massoli, P.; Ziemba, L.D.; Crosbie, E.C.; Moore, R.H.; et al. Substantial Seasonal Contribution of Observed Biogenic Sulfate Particles to Cloud Condensation Nuclei. Sci. Rep. 2018, 8, 3235. [Google Scholar] [CrossRef]
- Korhonen, H.; Carslaw, K.S.; Spracklen, D.V.; Mann, G.W.; Woodhouse, M.T. Influence of oceanic dimethyl sulfide emissions on cloud condensation nuclei concentrations and seasonality over the remote Southern Hemisphere oceans: A global model study. J. Geophys. Res.-Atmos. 2008, 113, D15204. [Google Scholar] [CrossRef]
- Xu, F.; Yan, S.-B.; Zhang, H.-H.; Wu, Y.-C.; Ma, Q.-Y.; Song, Y.-C.; Zhuang, G.-C.; Yang, G.-P. Occurrence and cycle of dimethyl sulfide in the western Pacific Ocean. Limnol. Oceanogr. 2021, 66, 2868–2884. [Google Scholar] [CrossRef]
- Vallina, S.M.; Simo, R. Strong Relationship Between DMS and the Solar Radiation Dose over the Global Surface Ocean. Science 2007, 315, 506–508. [Google Scholar] [CrossRef]
- Galí, M.; Saló, V.; Almeda, R.; Calbet, A.; Simó, R. Stimulation of gross dimethylsulfide (DMS) production by solar radiation. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Lawrence, M.G. An empirical analysis of the strength of the phytoplankton-dimethylsulfide-cloud-climate feedback cycle. J. Geophys. Res. 1993, 98, 20663–20673. [Google Scholar] [CrossRef]
- Foley, J.A.; Taylor, K.E.; Ghan, S.J. Planktonic dimethylsulfide and cloud albedo: An estimate of the feedback response. Clim. Chang. 1991, 18, 1–15. [Google Scholar] [CrossRef]
- Gabric, A.J.; Whetton, P.H.; Boers, R.; Ayers, G.P. The impact of simulated climate change on the air-sea flux of dimethylsulphide in the subantarctic Southern Ocean. Tellus B 1998, 50, 388–399. [Google Scholar] [CrossRef]
- Six, K.D.; Kloster, S.; Ilyina, T.; Archer, S.D.; Zhang, K.; Maier-Reimer, E. Global warming amplified by reduced sulphur fluxes as a result of ocean acidification. Nat. Clim. Chang. 2013, 3, 975–978. [Google Scholar] [CrossRef]
- Gunson, J.R.; Spall, S.A.; Anderson, T.R.; Jones, A.; Totterdell, I.J.; Woodage, M.J. Climate sensitivity to ocean dimethylsulphide emissions. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Vallina, S.M.; Simo, R.; Manizza, M. Weak response of oceanic dimethylsulfide to upper mixing shoaling induced by global warming. Proc. Natl. Acad. Sci. USA 2007, 104, 16004–16009. [Google Scholar] [CrossRef]
- Bock, J.; Michou, M.; Nabat, P.; Abe, M.; Mulcahy, J.P.; Olivie, D.J.L.; Schwinger, J.; Suntharalingam, P.; Tjiputra, J.; van Hulten, M.; et al. Evaluation of ocean dimethylsulfide concentration and emission in CMIP6 models. Biogeosciences 2021, 18, 3823–3860. [Google Scholar] [CrossRef]
- Szopa, S.; Naik, V.; Adhikary, B.; Artaxo, P.; Berntsen, T.; Collins, W.D.; Fuzzi, S.; Gallardo, L.; Kiendler-Scharr, A.; Klimont, Z. Short-Lived Climate Forcers. In Proceedings of the AGU Fall Meeting 2021, New Orleans, LA, USA, 3–17 December 2021. [Google Scholar]
- Cameron-Smith, P.; Elliott, S.; Maltrud, M.; Erickson, D.; Wingenter, O. Changes in dimethyl sulfide oceanic distribution due to climate change. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Gabric, A.J.; Simo, R.; Cropp, R.A.; Hirst, A.; Dachs, J. Modeling estimates of the global emission of dimethylsulfide under enhanced greenhouse conditions. Glob. Biogeochem. Cycle 2004, 18, GB2014. [Google Scholar] [CrossRef]
- Qu, B.; Gabric, A.J.; Jackson, R. Contemporary variability in dimethylsulfide flux in the Barents Sea and simulated change under 4×CO2 climate conditions. J. Mar. Syst. 2021, 220, 103573. [Google Scholar] [CrossRef]
- Qu, B.; Gabric, A.J.; Jackson, R. Simulated perturbation in the sea-to-air flux of dimethylsulfide and the impact on polar climate. J. Oceanol. Limnol. 2021, 39, 110–121. [Google Scholar] [CrossRef]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.-A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Cho, B.C.; Azam, F. Major role of bacteria in biogeochemical fluxes in the ocean’s interior. Nature 1988, 332, 441–443. [Google Scholar] [CrossRef]
- Pomeroy, L.R.; Williams, P.J.l.; Azam, F.; Hobbie, J.E. The microbial loop. Oceanography 2007, 20, 28–33. [Google Scholar] [CrossRef]
- Simó, R. Production of atmospheric sulfur by oceanic plankton: Biogeochemical, ecological and evolutionary links. Trends Ecol. Evol. 2001, 16, 287–294. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J.; Bruton, J.A. New and important roles for DMSP in marine microbial communities. J. Sea Res. 2000, 43, 209–224. [Google Scholar] [CrossRef]
- Cirri, E.; Pohnert, G. Algae-bacteria interactions that balance the planktonic microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Thume, K.; Gebser, B.; Chen, L.; Meyer, N.; Kieber, D.J.; Pohnert, G. The metabolite dimethylsulfoxonium propionate extends the marine organosulfur cycle. Nature 2018, 563, 412–415. [Google Scholar] [CrossRef]
- Dacey, J.W.H.; Wakeham, S.G. Oceanic dimethylsulfide: Production during zooplankton grazing on phytoplankton. Science 1986, 233, 1314–1316. [Google Scholar] [CrossRef]
- Laroche, D.; Vezina, A.; Levasseur, M.; Gosselin, M.; Stefels, J.; Keller, M.; Matrai, P.; Kwint, R. DMSP synthesis and exudation in phytoplankton: A modeling approach. Mar. Ecol. Prog. Ser. 1999, 180, 37–49. [Google Scholar] [CrossRef]
- Malin, G.; Wilson, W.H.; Bratbak, G.; Liss, P.S.; Mann, N.H. Elevated production of dimethylsulfide resulting from viral infection of cultures of Phaeocystis pouchetii. Limnol. Oceanogr. 1998, 43, 1389–1393. [Google Scholar] [CrossRef]
- Yoch, D.C. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Microbiol. 2002, 68, 5804–5815. [Google Scholar] [CrossRef]
- Groene, T. Biogenic production and consumption of dimethylsulfide (DMS) and dimethylsulfoniopropionate (DMSP) in the marine epipelagic zone: A review. J. Mar. Syst. 1995, 6, 191–209. [Google Scholar] [CrossRef]
- Kiene, R.P.; Bates, T.S. Biological removal of dimethylsulfide from seawater. Nature 1990, 345, 702–705. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Levine, N.M. Putting the spotlight on organic sulfur. Science 2016, 354, 418–419. [Google Scholar] [CrossRef]
- Moran, M.A.; Durham, B.P. Sulfur metabolites in the pelagic ocean. Nat. Rev. Microbiol. 2019, 17, 665–678. [Google Scholar] [CrossRef]
- Ksionzek, K.B.; Lechtenfeld, O.J.; McCallister, S.L.; Schmitt-Kopplin, P.; Geuer, J.K.; Geibert, W.; Koch, B.P. Dissolved organic sulfur in the ocean: Biogeochemistry of a petagram inventory. Science 2016, 354, 456–459. [Google Scholar] [CrossRef]
- Galí, M.; Devred, E.; Levasseur, M.; Royer, S.-J.; Babin, M. A remote sensing algorithm for planktonic dimethylsulfoniopropionate (DMSP) and an analysis of global patterns. Remote Sens. Environ. 2015, 171, 171–184. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef]
- Gao, C.; Fernandez, V.I.; Lee, K.S.; Fenizia, S.; Pohnert, G.; Seymour, J.R.; Raina, J.-B.; Stocker, R. Single-cell bacterial transcription measurements reveal the importance of dimethylsulfoniopropionate (DMSP) hotspots in ocean sulfur cycling. Nat. Commun. 2020, 11, 1942. [Google Scholar] [CrossRef]
- Reisch, C.R.; Moran, M.A.; Whitman, W.B. Dimethylsulfoniopropionate-Dependent Demethylase (DmdA) from Pelagibacter ubique and Silicibacter pomeroyi. J. Bacteriol. 2008, 190, 8018–8024. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J. Distribution and turnover of dissolved DMSP and its relationship with bacterial production and dimethylsulfide in the Gulf of Mexico. Limnol. Oceanogr. 2000, 45, 849–861. [Google Scholar] [CrossRef]
- Kiene, R.P.; Nowinski, B.; Esson, K.; Preston, C.; Marin III, R.; Birch, J.; Scholin, C.; Ryan, J.; Moran, M.A. Unprecedented DMSP concentrations in a massive dinoflagellate bloom in Monterey Bay, CA. Geophys. Res. Lett. 2019, 46, 12279–12288. [Google Scholar] [CrossRef]
- Stefels, J.; Steinke, M.; Turner, S.; Malin, G.; Belviso, S. Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 2007, 83, 245–275. [Google Scholar] [CrossRef]
- Novak, G.A.; Kilgour, D.B.; Jernigan, C.M.; Vermeuel, M.P.; Bertram, T.H. Oceanic emissions of dimethyl sulfide and methanethiol and their contribution to sulfur dioxide production in the marine atmosphere. Atmos. Chem. Phys. 2022, 22, 6309–6325. [Google Scholar] [CrossRef]
- Li, C.; Yang, G.-P.; Kieber, D.J.; Motard-Côté, J.; Kiene, R.P. Assessment of DMSP turnover reveals a non-bioavailable pool of dissolved DMSP in coastal waters of the Gulf of Mexico. Environ. Chem. 2016, 13, 266–279. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Liu, J.; Zhong, H.; Williams, B.T.; Zheng, Y.; Curson, A.R.J.; Sun, C.; Sun, H.; Song, D.; et al. Bacterial Dimethylsulfoniopropionate Biosynthesis in the East China Sea. Microorganisms 2021, 9, 657. [Google Scholar] [CrossRef]
- Zhuang, G.-C.; Lin, Y.-S.; Bowles, M.W.; Heuer, V.B.; Lever, M.A.; Elvert, M.; Hinrichs, K.-U. Distribution and isotopic composition of trimethylamine, dimethylsulfide and dimethylsulfoniopropionate in marine sediments. Mar. Chem. 2017, 196, 35–46. [Google Scholar] [CrossRef]
- Kasamatsu, N.; Kawaguchi, S.; Watanabe, S.; Odate, T.; Fukuchi, M. Possible impacts of zooplankton grazing on dimethylsulfide production in the Antartic Ocean. Can. J. Fish. Aquat. Sci. 2011, 61, 736–743. [Google Scholar] [CrossRef]
- Giovannoni, S.J. SAR11 Bacteria: The Most Abundant Plankton in the Oceans. Annu. Rev. Mar. Sci. 2017, 9, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Tripp, H.J.; Kitner, J.B.; Schwalbach, M.S.; Dacey, J.W.H.; Wilhelm, L.J.; Giovannoni, S.J. SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 2008, 452, 741–744. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; McParland, E.L.; Bramucci, A.R.; Siboni, N.; Ostrowski, M.; Kahlke, T.; Levine, N.M.; Brown, M.V.; van de Kamp, J.; Bodrossy, L.; et al. Biogeographical and seasonal dynamics of the marine Roseobacter community and ecological links to DMSP-producing phytoplankton. ISME Commun. 2022, 2, 16. [Google Scholar] [CrossRef]
- Durham, B.P.; Boysen, A.K.; Carlson, L.T.; Groussman, R.D.; Heal, K.R.; Cain, K.R.; Morales, R.L.; Coesel, S.N.; Morris, R.M.; Ingalls, A.E.; et al. Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat. Microbiol. 2019, 4, 1706–1715. [Google Scholar] [CrossRef]
- Howard, E.C.; Henriksen, J.R.; Buchan, A.; Reisch, C.R.; Bürgmann, H.; Welsh, R.; Ye, W.; González, J.M.; Mace, K.; Joye, S.B.; et al. Bacterial Taxa That Limit Sulfur Flux from the Ocean. Science 2006, 314, 649–652. [Google Scholar] [CrossRef]
- Seymour, J.R.; Amin, S.A.; Raina, J.-B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton–bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Vila-Costa, M.; Rinta-Kanto, J.M.; Poretsky, R.S.; Sun, S.; Kiene, R.P.; Moran, M.A. Microbial controls on DMSP degradation and DMS formation in the Sargasso Sea. Biogeochemistry 2014, 120, 295–305. [Google Scholar] [CrossRef]
- Motard-Côté, J.; Levasseur, M.; Scarratt, M.; Michaud, S.; Gratton, Y.; Rivkin, R.B.; Keats, K.; Gosselin, M.; Tremblay, J.É.; Kiene, R.P. Distribution and metabolism of dimethylsulfoniopropionate (DMSP) and phylogenetic affiliation of DMSP-assimilating bacteria in northern Baffin Bay/Lancaster Sound. J. Geophys. Res. Ocean. 2012, 117. [Google Scholar] [CrossRef]
- Lizotte, M.; Levasseur, M.; Law, C.S.; Walker, C.F.; Safi, K.A.; Marriner, A.; Kiene, R.P. Dimethylsulfoniopropionate (DMSP) and dimethyl sulfide (DMS) cycling across contrasting biological hotspots of the New Zealand subtropical front. Ocean Sci. 2017, 13, 961–982. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, S.-H.; Tian, J.-Y.; Zhang, Z.-Y.; Zhao, L.-J.; Xu, R.; Yang, G.-P.; Lai, J.-G.; Wang, X.-D. Distribution and Dimethylsulfoniopropionate Degradation of Dimethylsulfoniopropionate-Consuming Bacteria in the Yellow Sea and East China Sea. J. Geophys. Res. Ocean. 2021, 126, e2021JC017679. [Google Scholar] [CrossRef]
- Spiese, C.E.; Kieber, D.J.; Nomura, C.T.; Kiene, R.P. Reduction of dimethylsulfoxide to dimethylsulfide by marine phytoplankton. Limnol. Oceanogr. 2009, 54, 560–570. [Google Scholar] [CrossRef]
- Dixon, J.L.; Hopkins, F.E.; Stephens, J.A.; Schäfer, H. Seasonal Changes in Microbial Dissolved Organic Sulfur Transformations in Coastal Waters. Microorganisms 2020, 8, 337. [Google Scholar] [CrossRef]
- Zubkov, M.V.; Fuchs, B.M.; Archer, S.D.; Kiene, R.P.; Amann, R.; Burkill, P.H. Rapid turnover of dissolved DMS and DMSP by defined bacterioplankton communities in the stratified euphotic zone of the North Sea. Deep-Sea Res. Part II-Top. Stud. Oceanogr. 2002, 49, 3017–3038. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; Duarte, C.M. Respiration in the open ocean. Nature 2002, 420, 379–384. [Google Scholar] [CrossRef]
- Dutkiewicz, S.; Scott, J.R.; Follows, M.J. Winners and losers: Ecological and biogeochemical changes in a warming ocean. Glob. Biogeochem. Cycle 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Barton, S.; Yvon-Durocher, G. Quantifying the temperature dependence of growth rate in marine phytoplankton within and across species. Limnol. Oceanogr. 2019, 64, 2081–2091. [Google Scholar] [CrossRef]
- Schabhüttl, S.; Hingsamer, P.; Weigelhofer, G.; Hein, T.; Weigert, A.; Striebel, M. Temperature and species richness effects in phytoplankton communities. Oecologia 2013, 171, 527–536. [Google Scholar] [CrossRef]
- Ullah, H.; Nagelkerken, I.; Goldenberg, S.U.; Fordham, D.A. Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PLoS Biol. 2018, 16, e2003446. [Google Scholar] [CrossRef]
- Anderson, S.I.; Barton, A.D.; Clayton, S.; Dutkiewicz, S.; Rynearson, T.A. Marine phytoplankton functional types exhibit diverse responses to thermal change. Nat. Commun. 2021, 12, 6413. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Legendre, L. Biogenic carbon cycling in the upper ocean: Effects of microbial respiration. Science 2001, 291, 2398–2400. [Google Scholar] [CrossRef]
- López-Urrutia, Á.; Martin, E.S.; Harris, R.P.; Irigoien, X. Scaling the metabolic balance of the oceans. Proc. Natl. Acad. Sci. USA 2006, 103, 8739–8744. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M.; Shiah, F.K.; Smith, E.M. Microbial processes and temperature in Chesapeake Bay: Current relationships and potential impacts of regional warming. Glob. Chang. Biol. 2002, 8, 51–70. [Google Scholar] [CrossRef]
- Ducklow, H.W.; Morán, X.A.G.; Murray, A.E. Bacteria in the greenhouse: Marine microbes and climate change. In Environmental Microbiology; Mitchell, R., Ji-Dong Gu, J.-D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–31. [Google Scholar]
- Vaqué, D.; Lara, E.; Arrieta, J.M.; Holding, J.; Sà, E.L.; Hendriks, I.E.; Coello-Camba, A.; Alvarez, M.; Agustí, S.; Wassmann, P.F.; et al. Warming and CO2 Enhance Arctic Heterotrophic Microbial Activity. Front. Microbiol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.; Heinze, C.; Ilyina, T.; Seferian, R.; et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Lewandowska, A.M.; Boyce, D.G.; Hofmann, M.; Matthiessen, B.; Sommer, U.; Worm, B. Effects of sea surface warming on marine plankton. Ecol. Lett. 2014, 17, 614–623. [Google Scholar] [CrossRef]
- Wohlers, J.; Engel, A.; Zollner, E.; Breithaupt, P.; Jurgens, K.; Hoppe, H.G.; Sommer, U.; Riebesell, U. Changes in biogenic carbon flow in response to sea surface warming. Proc. Natl. Acad. Sci. USA 2009, 106, 7067–7072. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Goldenberg, S.U.; Ferreira, C.M.; Ullah, H.; Connell, S.D. Trophic pyramids reorganize when food web architecture fails to adjust to ocean change. Science 2020, 369, 829–832. [Google Scholar] [CrossRef]
- Sarmento, H.; Montoya, J.M.; Vázquez-Domínguez, E.; Vaqué, D.; Gasol, J.M. Warming effects on marine microbial food web processes: How far can we go when it comes to predictions? Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2137–2149. [Google Scholar] [CrossRef]
- Segschneider, J.; Bendtsen, J. Temperature-dependent remineralization in a warming ocean increases surface pCO2 through changes in marine ecosystem composition. Glob. Biogeochem. Cycle 2013, 27, 1214–1225. [Google Scholar] [CrossRef]
- Armstrong McKay, D.I.; Cornell, S.E.; Richardson, K.; Rockström, J. Resolving ecological feedbacks on the ocean carbon sink in Earth system models. Earth Syst. Dynam. 2021, 12, 797–818. [Google Scholar] [CrossRef]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef]
- Pomeroy, L.R.; Wiebe, W.J. Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat. Microb. Ecol. 2001, 23, 187–204. [Google Scholar] [CrossRef]
- Brewer, P.G.; Peltzer, E.T. Depth perception: The need to report ocean biogeochemical rates as functions of temperature, not depth. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160319. [Google Scholar] [CrossRef] [PubMed]
- Gabric, A.J.; Gregg, W.; Najarr, R.; Erickson, D.; Matrai, P. Modelling the biogeochemical cycle of dimethylsulphide in the upper ocean. Chemosphere Glob. Chang. Sci. 2001, 3, 377–392. [Google Scholar] [CrossRef]
- Moloney, C.L.; Field, J.G. Size-based dynamics of plankton food webs. I. A simulation model of carbon and nitrogen flows. J. Plankton Res. 1991, 13, 1003–1038. [Google Scholar] [CrossRef]
- Gabric, A.J.; Matrai, P.; Vernet, M. Modelling the production of dimethylsulphide during the vernal bloom in the Barents Sea. Tellus B 1999, 51, 919–938. [Google Scholar] [CrossRef]
- Qu, B.; Gabric, A.J. Using genetic algorithms to calibrate a dimethylsulfide production model in the Arctic Ocean. Chin. J. Oceanol. Limnol. 2010, 28, 573–582. [Google Scholar] [CrossRef]
- Gabric, A.J.; Whetton, P.; Cropp, R. Dimethylsulphide production in the subantarctic Southern Ocean under enhanced greenhouse conditions. Tellus B 2001, 53, 273–287. [Google Scholar] [CrossRef]
- Watts, M.; Bigg, G. Modelling the nitrogen cycle and DMS production in Lagrangian experiments in the North Atlantic. Deep.-Sea Res. II 2001, 48, 1019–1042. [Google Scholar] [CrossRef]
- Qu, B.; Zhao, L.; Gabric, A.J. Simulating the sea-to-air flux of dimethylsulfide sea in the eastern China marginal seas. J. Mar. Syst. 2020, 212, 103450. [Google Scholar] [CrossRef]
- Qu, B.; Gabric, A.J.; Zeng, M.; Lu, Z. Dimethylsulfide model calibration in the Barents Sea using a genetic algorithm and neural network. Environ. Chem. 2015, 13, 413–424. [Google Scholar] [CrossRef]
- Halloran, P.R.; Bell, T.G.; Totterdell, I.J. Can we trust empirical marine DMS parameterisations within projections of future climate? Biogeosciences 2010, 7, 1645–1656. [Google Scholar] [CrossRef]
- Hind, A.J.; Rauschenberg, C.D.; Johnson, J.E.; Yang, M.; Matrai, P.A. The use of algorithms to predict surface seawater dimethyl sulphide concentrations in the SE Pacific, a region of steep gradients in primary productivity, biomass and mixed layer depth. Biogeosciences 2011, 8, 1–16. [Google Scholar] [CrossRef]
- Anderson, T.R.; Spall, S.A.; Yool, A.; Cipollini, P.; Challenor, P.G.; Fasham, M.J.R. Global fields of sea surface dimethylsulfide predicted from chlorophyll, nutrients and light. J. Mar. Syst. 2001, 30, 1–20. [Google Scholar] [CrossRef]
- Galí, M.; Levasseur, M.; Devred, E.; Simó, R.; Babin, M. Sea-surface dimethylsulfide (DMS) concentration from satellite data at global and regional scales. Biogeosciences 2018, 15, 3497–3519. [Google Scholar] [CrossRef]
- Herr, A.E.; Kiene, R.P.; Dacey, J.W.H.; Tortell, P.D. Patterns and drivers of dimethylsulfide concentration in the northeast subarctic Pacific across multiple spatial and temporal scales. Biogeosciences 2019, 16, 1729–1754. [Google Scholar] [CrossRef]
- McNabb, B.J.; Tortell, P.D. Improved prediction of dimethyl sulfide (DMS) distributions in the northeast subarctic Pacific using machine-learning algorithms. Biogeosciences 2022, 19, 1705–1721. [Google Scholar] [CrossRef]
- Tesdal, J.-E.; Christian, J.R.; Monahan, A.H.; von Salzen, K. Evaluation of diverse approaches for estimating sea-surface DMS concentration and air–sea exchange at global scale. Environ. Chem. 2016, 13, 390–412. [Google Scholar] [CrossRef]
- Gabric, A.J.; Cropp, R.A.; Hirst, A.C.; Marchant, H.J. The response of dimethylsulphide production to simulated warming in the eastern Antarctic Southern Ocean. Tellus B 2003, 55, 966–981. [Google Scholar] [CrossRef]
- Gabric, A.J.; Qu, B.; Matrai, P.; Hirst, A.C. The simulated response of dimethylsulfide production in the Arctic Ocean to global warming. Tellus B 2005, 57, 391–403. [Google Scholar] [CrossRef]
- Bopp, L.; Aumont, O.; Belviso, S.; Monfray, P. Potential impact of climate change on marine dimethyl sulfide emissions. Tellus Ser. B-Chem. Phys. Meteorol. 2003, 55, 11–22. [Google Scholar] [CrossRef]
- Wang, S.; Maltrud, M.; Elliott, S.; Cameron-Smith, P.; Jonko, A. Influence of dimethyl sulfide on the carbon cycle and biological production. Biogeochemistry 2018, 138, 49–68. [Google Scholar] [CrossRef]
- Hopkins, F.E.; Suntharalingam, P.; Gehlen, M.; Andrews, O.; Archer, S.D.; Bopp, L.; Buitenhuis, E.; Dadou, I.; Duce, R.; Goris, N.; et al. The impacts of ocean acidification on marine trace gases and the implications for atmospheric chemistry and climate. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20190769. [Google Scholar] [CrossRef]
- Taucher, J.; Oschlies, A. Can we predict the direction of marine primary production change under global warming? Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Laufkötter, C.; Vogt, M.; Gruber, N.; Aita-Noguchi, M.; Aumont, O.; Bopp, L.; Buitenhuis, E.; Doney, S.C.; Dunne, J.; Hashioka, T.; et al. Drivers and uncertainties of future global marine primary production in marine ecosystem models. Biogeosciences 2015, 12, 6955–6984. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Avgoustidi, V.; Nightingale, P.D.; Joint, I.; Steinke, M.; Turner, S.M.; Hopkins, F.E.; Liss, P.S. Decreased marine dimethyl sulfide production under elevated CO2 levels in mesocosm and in vitro studies. Environ. Chem. 2012, 9, 399–404. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Pohnert, G. Influence of temperature and elevated carbon dioxide on the production of dimethylsulfoniopropionate and glycine betaine by marine phytoplankton. Mar. Environ. Res. 2012, 73, 62–69. [Google Scholar] [CrossRef]
- Arnold, H.E.; Kerrison, P.; Steinke, M. Interacting effects of ocean acidification and warming on growth and DMS-production in the haptophyte coccolithophore Emiliania huxleyi. Glob. Chang. Biol. 2013, 19, 1007–1016. [Google Scholar] [CrossRef]
- Park, K.-T.; Lee, K.; Shin, K.; Yang, E.J.; Hyun, B.; Kim, J.-M.; Noh, J.H.; Kim, M.; Kong, B.; Choi, D.H.; et al. Direct Linkage between Dimethyl Sulfide Production and Microzooplankton Grazing, Resulting from Prey Composition Change under High Partial Pressure of Carbon Dioxide Conditions. Environ. Sci. Technol. 2014, 48, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, F.E.; Archer, S.D. Consistent increase in dimethyl sulfide (DMS) in response to high CO2 in five shipboard bioassays from contrasting NW European waters. Biogeosciences 2014, 11, 4925–4940. [Google Scholar] [CrossRef]
- Li, P.-F.; Yang, G.-P.; Liu, C.-Y. Combined effects of elevated temperature and pCO2 on the production of DMSP and DMS in the culture of Amphidinium carterae. J. Appl. Phycol. 2020, 32, 1063–1074. [Google Scholar] [CrossRef]
- Saint-Macary, A.D.; Barr, N.; Armstrong, E.; Safi, K.; Marriner, A.; Gall, M.; McComb, K.; Dillingham, P.W.; Law, C.S. The Influence of Ocean Acidification and Warming on DMSP & DMS in New Zealand Coastal Water. Atmosphere 2021, 12, 181. [Google Scholar]
- Bénard, R.; Levasseur, M.; Scarratt, M.; Michaud, S.; Starr, M.; Mucci, A.; Ferreyra, G.; Gosselin, M.; Tremblay, J.É.; Lizotte, M.; et al. Contrasting effects of acidification and warming on dimethylsulfide concentrations during a temperate estuarine fall bloom mesocosm experiment. Biogeosciences 2019, 16, 1167–1185. [Google Scholar] [CrossRef]
- Benard, R.; Lizotte, M.; Levasseur, M.; Scarratt, M.; Michaud, S.; Starr, M.; Tremblay, J.E.; Kiene, R.P.; Kameyama, S. Impact of anthropogenic pH perturbation on dimethyl sulfide cycling: A peek into the microbial black box. Elem.-Sci. Anthr. 2021, 9, 00043. [Google Scholar] [CrossRef]
- Hopkins, F.E.; Turner, S.M.; Nightingale, P.D.; Steinke, M.; Bakker, D.; Liss, P.S. Ocean acidification and marine trace gas emissions. Proc. Natl. Acad. Sci. USA 2010, 107, 760–765. [Google Scholar] [CrossRef]
- Dix, M.; Bi, D.; Dobrohotoff, P.; Fiedler, R.; Harman, I.; Law, R.; Mackallah, C.; Marsland, S.; O’Farrell, S.; Rashid, H.; et al. CSIRO-ARCCSS ACCESS-CM2 Model Output Prepared for CMIP6. Earth System Grid Federation. 2019. Available online: https://www.wdc-climate.de/ui/cmip6?input=CMIP6.ScenarioMIP.CSIRO-ARCCSS.ACCESS-CM2.ssp245 (accessed on 26 July 2022). [CrossRef]
- Krasting, J.P.; John, J.G.; Blanton, C.; McHugh, C.; Nikonov, S.; Radhakrishnan, A.; Rand, K.; Zadeh, N.T.; Balaji, V.; Durachta, J.; et al. NOAA-GFDL GFDL-ESM4 Model Output Prepared for CMIP6. Earth System Grid Federation. 2018. Available online: https://www.wdc-climate.de/ui/cmip6?input=CMIP6.CMIP.NOAA-GFDL.GFDL-ESM4 (accessed on 26 July 2022). [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Fricko, O.; Havlik, P.; Rogelj, J.; Klimont, Z.; Gusti, M.; Johnson, N.; Kolp, P.; Strubegger, M.; Valin, H.; Amann, M. The marker quantification of the Shared Socioeconomic Pathway 2: A middle-of-the-road scenario for the 21st century. Glob. Environ. Chang. 2017, 42, 251–267. [Google Scholar] [CrossRef]
- Kriegler, E.; Bauer, N.; Popp, A.; Humpenöder, F.; Leimbach, M.; Strefler, J.; Baumstark, L.; Bodirsky, B.L.; Hilaire, J.; Klein, D. Fossil-fueled development (SSP5): An energy and resource intensive scenario for the 21st century. Glob. Environ. Chang. 2017, 42, 297–315. [Google Scholar] [CrossRef]
- IPPC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Core Writing Team; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Gabric, A.; Matrai, P.; Jones, G.; Middleton, J. The nexus between sea ice and polar emissions of marine biogenic aerosols. Bull. Am. Meteorol. Soc. 2018, 99, 61–82. [Google Scholar] [CrossRef]
- Asher, E.C.; Dacey, J.W.H.; Stukel, M.; Long, M.C.; Tortell, P.D. Processes driving seasonal variability in DMS, DMSP, and DMSO concentrations and turnover in coastal Antarctic waters. Limnol. Oceanogr. 2017, 62, 104–124. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Kirchman, D.L.; Morán, X.A.G.; Ducklow, H. Microbial growth in the polar oceans—Role of temperature and potential impact of climate change. Nat. Rev. Microbiol. 2009, 7, 451–459. [Google Scholar] [CrossRef]
- Kim, H.; Ducklow, H.W. A Decadal (2002–2014) Analysis for Dynamics of Heterotrophic Bacteria in an Antarctic Coastal Ecosystem: Variability and Physical and Biogeochemical Forcings. Front. Mar. Sci. 2016, 3, 214. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Shadwick, E.H.; Trull, T.W.; Thomas, H.; Gibson, J.A.E. Vulnerability of Polar Oceans to Anthropogenic Acidification: Comparison of Arctic and Antarctic Seasonal Cycles. Sci. Rep. 2013, 3, 2339. [Google Scholar] [CrossRef]
- Tynan, E.; Clarke, J.S.; Humphreys, M.P.; Ribas-Ribas, M.; Esposito, M.; Rérolle, V.M.C.; Schlosser, C.; Thorpe, S.E.; Tyrrell, T.; Achterberg, E.P. Physical and biogeochemical controls on the variability in surface pH and calcium carbonate saturation states in the Atlantic sectors of the Arctic and Southern Oceans. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 127, 7–27. [Google Scholar] [CrossRef]
- Hussherr, R.; Levasseur, M.; Lizotte, M.; Tremblay, J.É.; Mol, J.; Thomas, H.; Gosselin, M.; Starr, M.; Miller, L.A.; Jarniková, T.; et al. Impact of ocean acidification on Arctic phytoplankton blooms and dimethyl sulfide concentration under simulated ice-free and under-ice conditions. Biogeosciences 2017, 14, 2407–2427. [Google Scholar] [CrossRef]
- Archer, S.D.; Kimmance, S.A.; Stephens, J.A.; Hopkins, F.E.; Bellerby, R.G.J.; Schulz, K.G.; Piontek, J.; Engel, A. Contrasting responses of DMS and DMSP to ocean acidification in Arctic waters. Biogeosciences 2013, 10, 1893–1908. [Google Scholar] [CrossRef]
- Hopkins, F.E.; Nightingale, P.D.; Stephens, J.A.; Moore, C.M.; Richier, S.; Cripps, G.L.; Archer, S.D. Dimethylsulfide (DMS) production in polar oceans may be resilient to ocean acidification. Biogeosci. Discuss 2018, 10, 1–42. [Google Scholar]
- Hopkins, F.E.; Nightingale, P.D.; Stephens, J.A.; Moore, C.M.; Richier, S.; Cripps, G.L.; Archer, S.D. A meta-analysis of microcosm experiments shows that dimethyl sulfide (DMS) production in polar waters is insensitive to ocean acidification. Biogeosciences 2020, 17, 163–186. [Google Scholar] [CrossRef]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; Van Hooidonk, R.; Eakin, C.M. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Chase, T.J.; Dietzel, A.; Hill, T.; Hoey, A.S.; Hoogenboom, M.O.; Jacobson, M. Global warming impairs stock–recruitment dynamics of corals. Nature 2019, 568, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; De’Ath, G.; Fabricius, K.E.; Lough, J.M. Declining coral calcification in massive Porites in two nearshore regions of the northern Great Barrier Reef. Glob. Chang. Biol. 2008, 14, 529–538. [Google Scholar] [CrossRef]
- Bourne, D.G.; Morrow, K.M.; Webster, N.S. Insights into the coral microbiome: Underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 2016, 70, 317–340. [Google Scholar] [CrossRef]
- Lesser, M.P. Coral bleaching: Causes and mechanisms. In Coral Reefs: An Ecosystem in Transition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 405–419. [Google Scholar]
- Berkelmans, R. Bleaching and mortality thresholds: How much is too much? In Coral Bleaching. Ecological Studies; van Oppen, M.J.H., Lough, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef]
- Dixon, A.M.; Forster, P.M.; Heron, S.F.; Stoner, A.M.K.; Beger, M. Future loss of local-scale thermal refugia in coral reef ecosystems. PLoS Clim. 2022, 1, e0000004. [Google Scholar] [CrossRef]
- Jackson, R.L.; Gabric, A.J.; Matrai, P.A.; Woodhouse, M.T.; Cropp, R.; Jones, G.B.; Deschaseaux, E.S.M.; Omori, Y.; McParland, E.L.; Swan, H.B. Parameterizing the impact of seawater temperature and irradiance on dimethylsulfide (DMS) in the Great Barrier Reef and the contribution of coral reefs to the global sulfur cycle. J. Geophys. Res. Ocean. 2021, 126, e012020JC016783. [Google Scholar] [CrossRef]
- Jackson, R.L.; Gabric, A.J.; Woodhouse, M.T.; Swan, H.B.; Jones, G.B.; Cropp, R.; Deschaseaux, E.S.M. Coral reef emissions of atmospheric dimethylsulfide and the influence on marine aerosols in the southern Great Barrier Reef, Australia. J. Geophys. Res. Atmos. 2020, 125, e032019JD031837. [Google Scholar] [CrossRef]
- Swan, H.B.; Jones, G.B.; Deschaseaux, E.S.M.; Eyre, B.D. Coral reef origins of atmospheric dimethylsulfide at Heron Island, southern Great Barrier Reef, Australia. Biogeosciences 2017, 14, 229–239. [Google Scholar] [CrossRef]
- Jones, G.B.; Curran, M.; Broadbent, A.; King, S.; Fischer, E.; Jones, R. Factors affecting the cycling of dimethylsulfide and dimethylsulfoniopropionate in coral reef waters of the Great Barrier Reef. Environ. Chem. 2007, 4, 310–322. [Google Scholar] [CrossRef]
- Jones, G.B.; Fischer, E.; Deschaseaux, E.S.M.; Harrison, P.L. The effect of coral bleaching on the cellular concentration of dimethylsulphoniopropionate in reef corals. J. Exp. Mar. Biol. Ecol. 2014, 460, 19–31. [Google Scholar] [CrossRef]
- Raina, J.-B.; Tapiolas, D.; Willis, B.L.; Bourne, D.G. Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl. Environ. Microbiol. 2009, 75, 3492–3501. [Google Scholar] [CrossRef]
- Deschaseaux, E.S.M.; Jones, G.B.; Deseo, M.A.; Shepherd, K.M.; Kiene, R.P.; Swan, H.B.; Harrison, P.L.; Eyre, B.D. Effects of environmental factors on dimethylated sulfur compounds and their potential role in the antioxidant system of the coral holobiont. Limnol. Oceanogr. 2014, 59, 758–768. [Google Scholar] [CrossRef]
- Gardner, S.G.; Nielsen, D.A.; Laczka, O.; Shimmon, R.; Beltran, V.H.; Ralph, P.J.; Petrou, K. Dimethylsulfoniopropionate, superoxide dismutase and glutathione as stress response indicators in three corals under short-term hyposalinity stress. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152418. [Google Scholar] [CrossRef]
- Jones, R.J.; Hoegh-Guldberg, O.; Larkum, A.W.D.; Schreiber, U. Temperature-induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 2002, 21, 1219–1230. [Google Scholar] [CrossRef]
- Lesser, M.P.; Stochaj, W.R.; Tapley, D.W.; Shick, J.M. Bleaching in coral reef anthozoans: Effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 1990, 8, 225–232. [Google Scholar] [CrossRef]
- Bay, L.K.; Doyle, J.; Logan, M.; Berkelmans, R. Recovery from bleaching is mediated by threshold densities of background thermo-tolerant symbiont types in a reef-building coral. R. Soc. Open Sci. 2016, 3, 160322. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.A.; Petrou, K.; Gates, R.D. Coral bleaching from a single cell perspective. ISME J. 2018, 12, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Berkelmans, R.; Van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’for coral reefs in an era of climate change. Proc. R. Soc. B Biol. Sci. 2006, 273, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.B.; King, S. Dimethylsulphoniopropionate (DMSP) as an indicator of bleaching tolerance in scleractinian corals. J. Mar. Sci. Eng. 2015, 3, 444–465. [Google Scholar] [CrossRef]
- Deschaseaux, E.S.M.; Beltran, V.H.; Jones, G.B.; Deseo, M.A.; Swan, H.B.; Harrison, P.L.; Eyre, B.D. Comparative response of DMS and DMSP concentrations in Symbiodinium clades C1 and D1 under thermal stress. J. Exp. Mar. Biol. Ecol. 2014, 459, 181–189. [Google Scholar] [CrossRef]
- Klueter, A.; Trapani, J.; Archer, F.I.; McIlroy, S.E.; Coffroth, M.A. Comparative growth rates of cultured marine dinoflagellates in the genus Symbiodinium and the effects of temperature and light. PLoS ONE 2017, 12, e0187707. [Google Scholar] [CrossRef]
- Jackson, R.; Gabric, A.; Cropp, R. Effects of ocean warming and coral bleaching on aerosol emissions in the Great Barrier Reef, Australia. Sci. Rep. 2018, 8, 14048. [Google Scholar] [CrossRef]
- Fischer, E.; Jones, G. Atmospheric dimethysulphide production from corals in the Great Barrier Reef and links to solar radiation, climate and coral bleaching. Biogeochemistry 2012, 110, 31–46. [Google Scholar] [CrossRef]
- Orr, J.C. Recent and future changes in ocean carbonate chemistry. Ocean. Acidif. 2011, 1, 41–66. [Google Scholar]
- Brodie, J.E.; Devlin, M.; Haynes, D.; Waterhouse, J. Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 2011, 106, 281–302. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- McKergow, L.A.; Prosser, I.P.; Hughes, A.O.; Brodie, J. Sources of sediment to the Great Barrier Reef world heritage area. Mar. Pollut. Bull. 2005, 51, 200–211. [Google Scholar] [CrossRef]
- Gabric, A.J.; Bell, P.R. Review of the effects of non-point nutrient loading on coastal ecosystems. Mar. Freshw. Res. 1993, 44, 261–283. [Google Scholar] [CrossRef]
- McCook, L.J.; Diaz-Pulido, G. The fate of bleached corals: Patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 2002, 232, 115–128. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 2010, 20, 840–850. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, R.; Gabric, A. Climate Change Impacts on the Marine Cycling of Biogenic Sulfur: A Review. Microorganisms 2022, 10, 1581. https://doi.org/10.3390/microorganisms10081581
Jackson R, Gabric A. Climate Change Impacts on the Marine Cycling of Biogenic Sulfur: A Review. Microorganisms. 2022; 10(8):1581. https://doi.org/10.3390/microorganisms10081581
Chicago/Turabian StyleJackson, Rebecca, and Albert Gabric. 2022. "Climate Change Impacts on the Marine Cycling of Biogenic Sulfur: A Review" Microorganisms 10, no. 8: 1581. https://doi.org/10.3390/microorganisms10081581





